ONO's Drug Reliability Assurance Activities
From a patient standpoint, ONO conducts drug reliability assurance activities with global perspective through drug life cycle to constantly check for drug quality assurance and reflect opinions from healthcare professionals and patients on further quality improvement. In addition, we analyze and assess, based on latest scientific evidence, drug quality and efficacy and safety (adverse reaction) information collected from, e.g., reports from patients and healthcare professionals, literature, and surveys, to constantly provide updates to the frontline of healthcare.
We are also a member of the following industry associations for the purpose of ensuring the quality and safety of our pharmaceutical products, and we promote reliability assurance activities in compliance with industry codes and guidelines regarding the proper use of pharmaceutical products:
- Japan Pharmaceutical Manufacturers Association
- Kansai Pharmaceutical Industries Association
- The Fair Trade Council of the Ethical Pharmaceutical Drugs Marketing Industry
- Council for the Proper Use of Drugs
- Japan Society of Quality Assurance
Quality Assurance Initiatives
We have established the “Quality Global Policy” as a company-wide policy on quality assurance, which clearly states our commitment to assuring quality, safety, and efficacy throughout the drug life cycle spanning from development to post-marketing, both in Japan and overseas, and we are committed to contributing to the safety and well-being of patients. Quality and safety risks are recognized as serious risks, which is why they are integrated and managed in company-wide ERM. In addition to legal requirements as a manufacturer and distributor of pharmaceutical products, we have also developed a quality manual in accordance with ICH guidelines to establish a global pharmaceutical quality system and are continuously working to improve our systems. Additionally, as a department responsible for internal audits of the quality management system, we have established the Global Quality Audit department within the Global Quality division, which is independent from the GxP implementation departments, to conduct internal audits as a third party. We also contribute to society by providing a stable supply of pharmaceuticals that are assured to a high-quality standard. As a result of these efforts, there were no recalls of ONO products in the fiscal year 2024.
In addition, to ensure that each and every employee attempts to raise their awareness of quality and safety, we regularly provide education on compliance with laws and regulations related to pharmaceuticals, as well as education on the harmful effects of drugs for all employees based on an appropriate management system which utilizes a system that appoints three responsible officers who are responsible for manufacturing and sales.
Initiatives for Proper Use of Pharmaceuticals
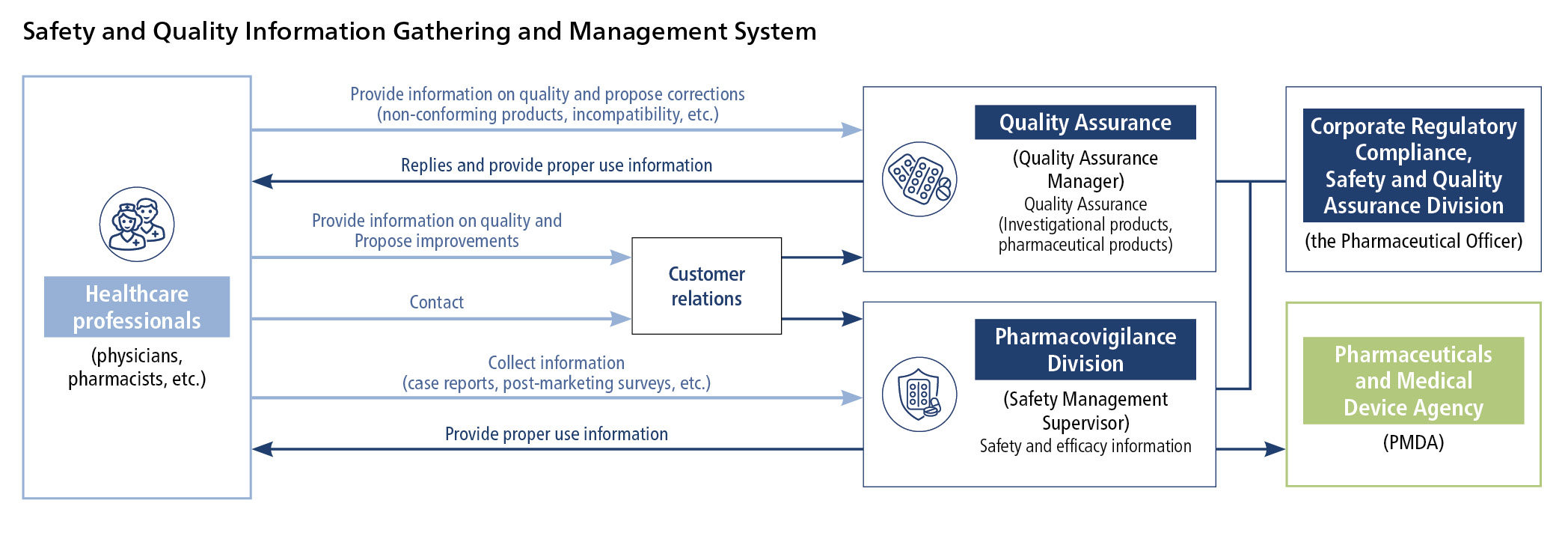
We develop a risk management plan and collect and manage safety (adverse reaction) information for each pharmaceutical.
We assess collected data and information, and if necessary revise the cautions on package inserts and make announcements about proper use.
As safety information drastically increases inside and outside Japan after market launch of antineoplastic drugs, we assess such information based on opinions from external medical experts to promote the proper use of the drugs, e.g., by disseminating it through promotional materials, conference presentations, and medical journals.
For safety management activities, too, we have created global standard procedure manual and databases and constructed a Group-wide system that extends to overseas operations.
Maintenance of Product Recall System
We have a system in place to recall any products with efficacy, quality or safety problems and to promptly provide medical professionals with information on them. We also have relevant departments jointly conduct periodical drills in preparation for product recall to check that they can be executed quickly even in unexpected circumstances.
